Uv-visible Spectroscopy Principle In Pdf
- Uv-visible Spectroscopy Principle In Pdf Print
- What Is Uv Visible Spectroscopy Pdf
- Uv-visible Spectroscopy Principle In Pdf Format
- Uv-visible Spectroscopy Principle In Pdf Format
- Uv-visible Spectroscopy Principle In Pdf Files
File Name: types of electronic transitions in uv spectroscopy creator.zip
Size: 28820Kb
Published: 09.08.2021
Electromagnetic spectrum. We see only a very small portion of the electromagnetic spectrum. In spectrochemical methods, we measure the absorption of UV to far IR radiation. UV = 200-380 nm VIS = 280-780 nm IR = 0.78 mm-300 mm ©Gary Christian, Analytical Chemistry, 6th Ed. Electromagnetic spectrum. We see only a very small portion of the electromagnetic spectrum. In spectrochemical methods, we measure the absorption of UV to far IR radiation. UV = 200-380 nm VIS = 280-780 nm IR = 0.78 mm-300 mm ©Gary Christian, Analytical Chemistry, 6th Ed.
Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. Fluorescence is brought about by absorption of photons in the singlet ground state promoted to a singlet excited state.
Uranium determination by UV-Vis spectrophotometry in organic matrix. Concentrations of uranium in the process samples provide essential information required for nuclear process monitoring. A large number of techniques have been developed to allow uranium determination, but each technique possesses some advantages and disadvantages and cannot be applied without difficulty to all samples.
Uv visible spectrophotometer principle pdf creator
Contents Introduction and principle Quantitative measurment and electronic Transitions Instrumentation and the experiment Applications Limitations. What is Spectroscopy?? Spectroscopyis the use of the absorption, emission, or scattering of electromagnetic radiation by matter to qualitatively or quantitatively study the matter or to study physical processes. The matter can be atoms, molecules, atomic or molecular ions, or solids. Types of spectroscopy Emission Spectroscopy: A method ofchemical analysisthat uses the intensity of light emitted from a flame or spark at a particular wavelength to determine the quantity of element in a sample.
Uv Visible Spectroscopy
Chapter 2 Instrumentation theory and techniques. Page 76 UV-visible spectra generally show only a few broad absorbance bands. Compared a count rate which is then output to a device such as a printer or computer monitor. The geometry of an evpv. Chapter 1 Principles and applications of UV-visible spectroscopy.
These metrics are regularly updated to reflect usage leading up to the last few days. Citations are the number of other articles citing this article, calculated by Crossref and updated daily. Find more information about Crossref citation counts. The Altmetric Attention Score is a quantitative measure of the attention that a research article has received online. Clicking on the donut icon will load a page at altmetric. Find more information on the Altmetric Attention Score and how the score is calculated.
Laser wavelengths ranging from ultra-violet through visible to near infra-red can be used for Raman spectroscopy. Typical examples include but are not limited to :. The choice of laser wavelength has an important impact on experimental capabilities:. Spatial resolution. For example, with a nm laser, and a 0. Thus, achievable spatial resolution is partially dependent on choice of laser. Ultra-violet UV lasers for Raman spectroscopy typically include laser wavelengths ranging from nm through to nm.
Uv visible spectrophotometer principle pdf creator. principle which states that Generally, the most probable transition uv vis absorption spectroscopy pdf is from. species in uv vis absorption spectroscopy pdf either solid or aqueous state.
UV-VIS Spectroscopy and Its Applications
За ее спиной ТРАНСТЕКСТ издал предсмертный оглушающий стон. Когда распался последний силиконовый чип, громадная раскаленная лава вырвалась наружу, пробив верхнюю крышку и выбросив на двадцать метров вверх тучу керамических осколков, и в то же мгновение насыщенный кислородом воздух шифровалки втянуло в образовавшийся вакуум. Сьюзан едва успела взбежать на верхнюю площадку лестницы и вцепиться в перила, когда ее ударил мощный порыв горячего ветра.
Нет сомнений, что человеческий мозг все же совершеннее самого быстродействующего компьютера в мире. В какую-то долю секунды сознание Беккера засекло очки в металлической оправе, обратилось к памяти в поисках аналога, нашло его и, подав сигнал тревоги, потребовало принять решение. Он отбросил бесполезный мотоцикл и пустился бежать со всех ног. К несчастью для Беккера, вместо неуклюжего такси Халохот обрел под ногами твердую почву. Спокойно подняв пистолет, он выстрелил.
Ну и. Но тебе там понравится. ГЛАВА 50 Фил Чатрукьян остановился в нескольких ярдах от корпуса ТРАНСТЕКСТА, там, где на полу белыми буквами было выведено: НИЖНИЕ ЭТАЖИ ШИФРОВАЛЬНОГО ОТДЕЛА ВХОД ТОЛЬКО ДЛЯ ЛИЦ СО СПЕЦИАЛЬНЫМ ДОПУСКОМ Чатрукьян отлично знал, что к этим лицам не принадлежит.

What is UV-visible spectroscopy PDF?
Ultraviolet- Visible Spectroscopy. Ultraviolet and visible (UV-Vis) absorption spectroscopy is the measurement of the. attenuation of a beam of light after it passes through a sample or after reflection from. a sample surface. The visible spectrum ranges from 400 nm to about 800 nm.
What is the basic principle of UV-Visible Spectroscopy?
The Principle of UV-Visible Spectroscopy is based on the absorption of ultraviolet light or visible light by chemical compounds, which results in the production of distinct spectra. Spectroscopy is based on the interaction between light and matter.
What is the basic difference between UV and visible spectroscopy?
100 – 900 nm range is known as far ultraviolet and 190 – 400 nm is known as near ultraviolet. Visible range extends from 400 – 800 nm. Most of the commercial instruments cover 180 – 800 nm region. The technique is known as molecular absorption spectrophotometry or spectrophotometry or simply colorimetry.
What is meant by UV-Visible Spectroscopy?
Ultraviolet–visible spectroscopy or ultraviolet–visible spectrophotometry (UV–Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. This means it uses light in the visible and adjacent ranges.
What is the purpose of UV Visible Spectroscopy?
UV/VIS/NIR spectroscopy is generally used to determine analyte concentrations or the chemical conversion of a component in solution. The technique measures the absorption of light across the desired optical range.
What is the purpose of UV spectroscopy?
UV-Vis Spectroscopy (or Spectrophotometry) is a quantitative technique used to measure how much a chemical substance absorbs light.
Which detector is used in UV spectroscopy?
photomultiplier tube
What are the detectors used in HPLC?
HPLC Detectors
- UV-Vis Detectors. The SPD-20A and SPD-20AV are general-purpose UV-Vis detectors offering an exceptional level of sensitivity and stability.
- Refractive Index Detector.
- Fluorescence Detectors.
- Evaporative Light Scattering Detector.
- Conductivity Detector.
What are the main parts of the spectrometer?
The spectrometer is an optical instrument used to study the spectra of different sources of light and to measure the refractive indices of materials (Fig. ). It consists of basically three parts. They are collimator, prism table and Telescope.১ এপ্রিল, ২০১৬
Which region of light wavelength is used in UV spectroscopy?
In UV/Vis/NIR spectroscopy the ultraviolet (170 nm to 380 nm), visible (380 nm to 780 nm), and near infrared (780 nm to 3300 nm) are used.
What is maximum absorbance?
(a) wavelength of maximum absorbance (λmax) The extent to which a sample absorbs light depends upon the wavelength of light. The wavelength at which a substance shows maximum absorbance is called absorption maximum or λmax.৩১ মার্চ, ২০১৬
Is benzene UV active?
Uv-visible Spectroscopy Principle In Pdf Print
Benzene exhibits very strong light absorption near 180 nm (ε > 65,000) , weaker absorption at 200 nm (ε = 8,000) and a group of much weaker bands at 254 nm (ε = 240).
Does benzene absorb UV light?
Note that both benzene and naphthacene absorb light in the near ultraviolet but that the latter does so much more intensely. A solution of naphthacene will absorb almost 100 times as much light at 250 nm. as a solution of benzene of the same molar concentration.
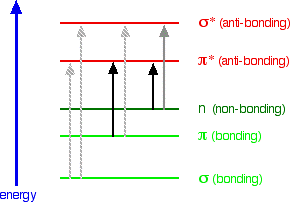
Which compound increases UV absorption?
Ag
How do you know if a compound is UV active?
Ones that do are said to be “UV-active” and ones that do not are “UV- inactive.” To be UV-active, compounds must possess a certain degree of conjugation, which occurs most commonly in aromatic compounds. One can then outline the spots with a pencil, while under the UV light, to mark their location.
What absorbs UV radiation?
As sunlight passes through the atmosphere, all UVC and most UVB is absorbed by ozone, water vapour, oxygen and carbon dioxide. Ozone is a particularly effective absorber of UV radiation. As the ozone layer gets thinner, the protective filter activity of the atmosphere is progressively reduced.৯ মার্চ, ২০১৬
How is UV generated?
UV radiation is produced either by heating a body to an incandescent temperature, as is the case with solar UV, or by passing an electric current through a gas, usually vaporized mercury. The latter process is the mechanism whereby UV radiation is produced artificially.
What is the major disadvantage of UV light as a disinfectant sterilant?
Disadvantages of UV disinfection? UV light needs the right amount of energy to be effective. UV light is effective for microorganisms not for chemicals. Photochemical damage caused by UV may be repaired by some organisms.২ জানু, ২০২০
How long does it take UV light to sterilize something?
30 minutes
Do hospitals use UV light?
The use of ultraviolet light systems is becoming more widely used in healthcare facilities for disinfecting patient and operating rooms. UV-C is also used in night time cleaning of laboratories and meat packing facilities. Another term used for UV-C disinfection is Ultraviolet Germicidal Irradiation (UVGI).
Does UV sanitizer really work?
That’s why UV light sanitizers can be a great alternative to products that might be out of stock. With any new technology, there will understandably be doubts about effectiveness, but research proves that in most cases UV sanitizers are effective in killing 99% of germs.১৪ নভেম্বর, ২০২০
What is the difference between ozone and UV?
What Is Uv Visible Spectroscopy Pdf
There is sometimes confusion as to the difference between ozone and UV systems. Ozone is dissolved in water to kill microorganisms, destroy organics, and break down chloramines by oxidation. In comparison, UV light inactivates microorganisms and breaks down chloramines with light energy.২৫ ফেব, ২০১৯
Is it safe to breathe ozone?
Uv-visible Spectroscopy Principle In Pdf Format
When inhaled, ozone can damage the lungs. Relatively low amounts can cause chest pain, coughing, shortness of breath and throat irritation. Ozone may also worsen chronic respiratory diseases such as asthma and compromise the ability of the body to fight respiratory infections.
Uv-visible Spectroscopy Principle In Pdf Format
Does UV light destroy ozone?
UV light will create ozone from atmospheric oxygen at short wavelengths of less than 240 nanometers (nm). UV light will also destroy ozone and break ozone back down into atomic oxygen (O) and diatomic oxygen (O2) at wavelengths from about 200 nm to 315 nm.
Does a UV light create ozone?
Uv-visible Spectroscopy Principle In Pdf Files
A UV lamp “tuned” to 185nm can create ozone from oxygen (O2) by disrupting the O2 molecule and splitting it into two oxygen atoms. These two oxygen atoms attempt to attach to other oxygen molecule (O2). It is the attachment of this third oxygen atom that creates ozone (O3).১ জুন, ২০২০